 Learn Metallurgy
Learn Metallurgymail@learnmetallurgy.com
Bayer process is a leaching process of bauxite. This process is use to produce high purity alumina (needed in subsequent electrolysis process) by leaching bauxite by NaOH. Leaching is done in autoclave at high presuure ( 25-30 atm) and high temperature (220 oC), which produces a soluble aluminate (2NaAlO2), and from which we precipitate out Al(OH)3. And finally alumina Al2O3 is produce by cacination of Al(OH)3.
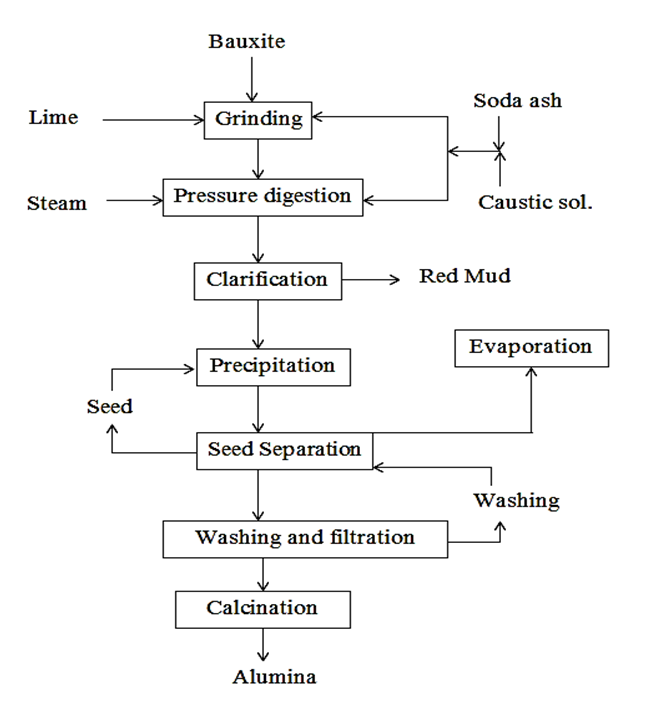
(1) Ore Preparation :
It is important to do ore preparation to reduce total precentage of silica in boxite. Using crushing and grinding, the silica content reduced from 10% to 1.5% as bauxite tends to get concentrated in the finer fractions (i.e., -100 mesh). Another importance of using finer bauxite is to increase total surface area which increases the rate of leaching.
(2) Digestion :
The fined-bauxite is mixed with caustic soda (NaOH) in a ball mill, and obtained slurry is fed into a digester (or autoclaves). The autoclaves are mentained at a tempurature of 220 oC and 25-30 atm pressure. The product obtained fron this process is calles as 'pregnant liqour' Reaction in autoclaves as-
\[ Al_2O_3.H_2O + NaOH \rightarrow 2NaAlO_2 + 2 H_2O \]
\[ Al_2O_3.3H_2O + NaOH \rightarrow 2NaAlO_2 + 4 H_2O \]
(3) Clarification :
The liqour obtained from digestion is cooled below 100 oC and after completely depressurizing liqour is taken to settling and clarification.Additive starch is added for settling of red mud. After settling, liqour is filtered through a series of filters. The final product is obtained from filteration is a clear colution of sodium aluminate (\( 2NaAlO_2 \) ), which leave behind residue like ferric hydroxide, silica and alumina.
\[ \textrm{Pregnant Liqour} \xrightarrow[\textrm{for precipitation}]{\textrm{Added Starch}} NaAlO_2 + \textrm{Red Mud} \]
Red mud :
Red mud contains \( Fe(OH)_2 , SiO_2, Al_2O_3 \). It is a low grade iron ore containing around 30-40% Fe. Addition to this, it also contains residual alkali which is not usable in blast furnace. Unusability of red mud make it a big problem for industries. There is no full proof use of red mud. Hence, industries dump red mud on huge land area and hoping in future we develop some methond to utilize it.
(4) Precipitation :
The filtrate is then cooled to temperature below its critical temperature for precipitating alumina. And small amount of very fine freshly prepared Al(OH)3 is added to start nucleation rocess. And result in precipitation of aluminium hydroxide (Al(OH)3).
\[ \textrm{Leach Liqour} \xrightarrow[f\textrm{or nucleation}]{Added Al(OH)_3} Al(OH)_3 \]
(5) Calcination :
Calcination is the final step in the Bayer process for production of metallurgical grade of alumina, which is done
either in rotary kilns or fluid bed stationary calciners by heating \( Al(OH)_3 \) above 1400 oC.
\[ Al(OH)_3 \xrightarrow[1400^o C]{\Delta} Al_2O_3 \]