Hall Heroult process is the commercialized smelting process of alumina for producing 99.5% pure aluminium metal. Hall Heroult is based on electrolytic decomposion of Al2O3 (Alumina) in a liquid bath of synthatic cryolite (Na3AlF6). Al2O3 is first dissolved in a carbon-lined bath of containing molten cryolite, Na3AlF6. Addition of aluminium fluoride, AlF3 is also done to reduce the melting point of the cryolite. At last, the mixture is electrolyzed, which reduces the liquid aluminium to bottom of bath.
The HH-process requires -
- A cheap electricity source
- High purity alumina (SiO2 < 5%)
- Cryolite
- Pre-backed ashless carbon electrodes
Schematic diagram of Hall-Heroult cell
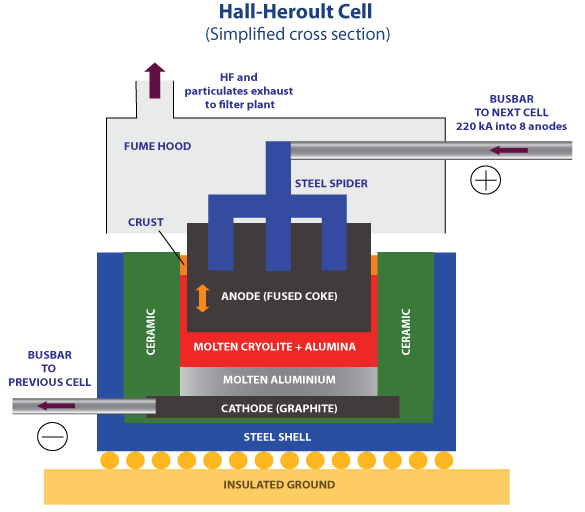
The above figure shows a Schematic diagram of electrolytic cell used for the electrolysis of alumina to produce aluminium. The cell contains -
- A rectangular refractory-lined steel boxes, having diamension of 5m long, 2m wide and 1m deep.
- The cathod lining are made up from ceramics.
- An iron plate and iron bar from which the cathode are embedded in the carbon mixure.
- A tap hole for collecting metal which is located at the bottom of the cell.
- The anodes which made from pre-backed carbon and have to replace continuously.
Cell operation :
- Firstly, the cell is filled with cryolite and then anode is lowered. And the current is passed through the cell circuit until the cryolite melts at temperature (960 -980 oC).
- After bath attains a molten state we start adding alumina to bath.
- Heat will generate because of resistance offered by both the electrodes and electrolyte. And the alumina will eventually decomposes to produce Al3+ and O2- ions.
- Al last the aluminium sinks to the bottom of the electrolytic cell, which is collected periodically in the span of 1 - 3 days and at the same time we feed alumina to the bath.
- Continuesly removal of Al and feeding of alumina is necessary to maintain the alumina content 5-10% in bath. If alumina content falls below 2%, it will cause anode effect.
- The bath aggitation is done by evolution of CO and CO2 gas and the magnetic stirring effect produced by the current flowing through the bath from the cathode lining. this helps to maintain fresh addition of alumina in bath.
Bath properties :
- The density of bath maintained such that it should facilitate Al sinking.
- Should have high ion conductivity but low e- conductivity. High conductivity will cause short circuit and no reduction of aluminium will take place.
- Apply high current instead of voltage for high heat generation.
Role of cryolite :
- Lowers the melting point of alumuna :
- Lowering density of bath :
- Electrolytic conductivity :
Anode effect
As we have seen earlier. it is very important to maintain 5 - 10% of alumina content in bath to facilitate easy electrolysis. But when alumina content in falls below 2% then alumina start reacting withh cryolite and forms gases like CF
4 and C
2Fe and, these get stuck on the electrodes. This results in distrupt the normal contact between anode and the bath hence, resistance between then abruptly increase and which increase voltage and cell operation ceases. This phenomenon is called anode effect.
Factor influencing electrolysis :
- Bath temperature :
The bath temperature is an factor with affect the current efficiency. In general, on every 4oC rise in temperature, the current efficiency falls by 1%. This happens due to the increase in numbser of side reaction which gets activated due to high temperature.
- Current density :
The current efficiency significantly increases on increasing the current density. Therefore, the current density is maintained at a very high value.
- Bath density :
On lowering the bath density, sinking ability of liberated aluminium will increase and hence, increasing seperation rate. The density of can be lowered by using AlF3/NaF ratio.
- Interpolar distance :
The interpolar distance is the distance between bottom of the anode and top of the molten bath. The current efficiency increases with increasing interpolar distance (In general, when distance is 65cm, current efficiency increases to 90%)
- Alumina content :
Strictly controlling the alumina content in bath, the cell efficiency can be improved. When the alumina content is 4%, the current efficiency touches it minima.
So maintaining Al2O3 below or at higher level can improve current efficience but it is advisable to maintain at higher percentage to avoid anode effect
 Learn Metallurgy
Learn Metallurgy