Crystal, any solid material in which array of atoms of solid are arranged in a orderly and periodic pattern and whose surface regularity reflects its internal symmetry.
Crystalline Vs Amorphous solids :
Crystalline are those material which exhibits 3D long range periodicity of arrangement of atom or molecules or ions. Crystallinity can be seen in metals, many ceramics and some polymer.
Amorphous have no periodic packing of atoms or molecules or ions. Occures from rapid cooling.
| CRYSTALLINE SOLIDS |
AMORPHOUS SOLIDS |
| Sharp melting point |
No particular melting point |
| Anisotropic |
Isotropic |
| True solid |
Pseudo solid |
| Symmetrical |
Unsymmetrical |
| More rigid |
Less rigid |
| Long range order |
Short range order |
| Example: Potassium nitrate, copper |
Example: Fibre Glass, PVC, Teflon etc. |
What is Lattice :
➧ A lattice is an infinite array of points
➧ Lattice is a translationally periodic arrangement of points
➧ Each lattice point is identical to every other point, i.e., the environment around one lattice site is identical to that around every other.
➧ Each point in the lattice is defined by \( R = n_{1}a_{1} + n_{2}a_{2} + n_{3}a_{3} \)
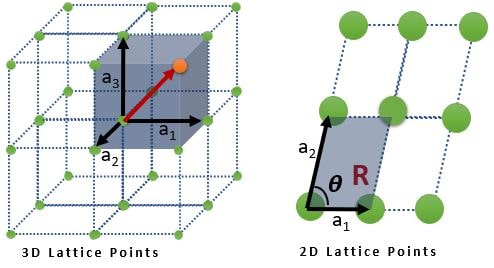
where \( n_{1} , n_{2} \textrm{ and } n_{3} \) is a set of three integers and \( a_{1} , a_{2} \textrm{ and } a_{3} \) are (known as the primitive vectors) three non coplanar vectors.
Unit Cell :
From Above figure, any parallelogram (and parallelopiped in 3D), connecting all lattice points is called a unit cell. There are infinite possibilities for unit cells.
In simple words The smallest repeating unit of the crystal lattice is the unit cell, and it should refect crystal's symmetry.
Types of Unit cell :
• Primitive Unit cell : Unit cell having one formula unit or one lattice point
• Non-primitive Unit cell : Unit Cell having more than one formula units or lattice points
Crystal Structure :
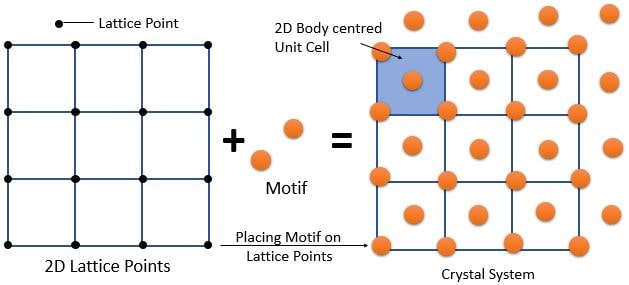
➧ Crystal structure can be constructed in many ways. Example : A Lattice and Motif (i.e., atom of group of atoms).
➧ There are many combinations of lattices and motifs possible that can result in the same crystal. There is no unique choice.
\[ \textrm{ Crystal Structure = Lattice + Motif } \]
Based on XRD technique, all crystalline materials are classified into 7-crystal system. And these 7-crystal system further divided into 14 Bravais lattices .
| Crystal System |
Geometry |
Crystal Symmetry |
Bravais Lattices |
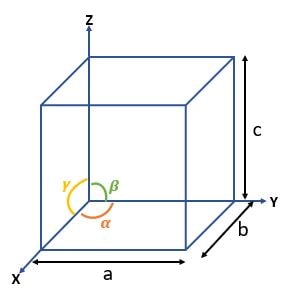
| Cube |
\( a = b = c \textrm{ ; } \alpha = \beta = \gamma = 90^\circ \) |
4 , 3-fold rotation axes  |
3 → SC, BCC, FCC |
| Tetragonal |
\( a = b \neq c \textrm{ ; } \alpha = \beta = \gamma = 90^\circ \) |
1 , 4-fold rotation axes |
2 → ST, BCT |
| Orthorhombic |
\( a \neq b \neq c \textrm{ ; } \alpha = \beta = \gamma = 90^\circ \) |
3 , \( \perp \) 2-fold rotation axes  |
4 → SO, BCO, FCO, ECO |
| Rhombohedral |
\( a = b = c \textrm{ ; } \alpha = \beta = \gamma \neq 90^\circ \) |
1 , 3-fold axis symmetry |
1 → SR |
| Hexagonal |
\( a = b \neq c \textrm{ ; } \alpha = \beta = 90^\circ \textrm{ ; } \gamma = 120^\circ \) |
1 , 6-fold rotation axes  |
2 → SH |
| Monoclinic |
\( a \neq b \neq c \textrm{ ; } \alpha = \beta = 90^\circ \neq \gamma \) |
1 , 2-fold rotation axes |
2 → SM, ECM |
| Triclinic |
J\( a \neq b \neq c \textrm{ ; } \alpha \neq \beta \neq \gamma \neq 90^\circ \) |
None |
1 → S.Triclinic |
|
|
Total Bravais Lattices : |
14 |
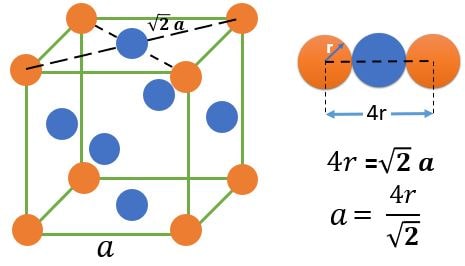
FCC (Face Centered Cubic) :
Atom Positions :
(1) 8 Atoms, At every corner contributing \( \frac{1}{8} \) => 8 Corner atoms \( \times \) \( \frac{1}{8} \) contribution = 1 atom
(2) 6 Atoms, At every face center contributing \( \frac{1}{2} \) => 6 Face centered atoms \( \times \) \( \frac{1}{2} \) contribution = 3 atom
Total number of atoms = 1+3 = 4 atoms.
 Notes :
Notes :
(1) FCC Elements are generally strong and ductile
(2) Examples :- \( \gamma-Fe \), Al, Cu, Ni, Au, Ag, Pt.
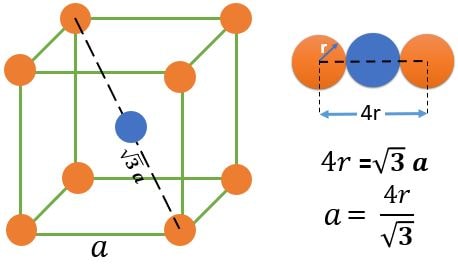
BCC (Base Centered Cubic) :
Atom Positions :
(1) 8 Atoms, At every corner contributing \( \frac{1}{8} \) => 8 Corner atoms \( \times \) \( \frac{1}{8} \) contribution = 1 atom
(2) 1 Atoms, At every Body center contributing 1 => 1 atom
Total number of atoms = 1+1 = 2 atoms.
 Notes :
Notes :
(1) BCC Elements are generally hard and brittle
(2) Examples :- \( \alpha-Fe \), \( \delta-Fe \), W, Cr, V, Mo, Ta.
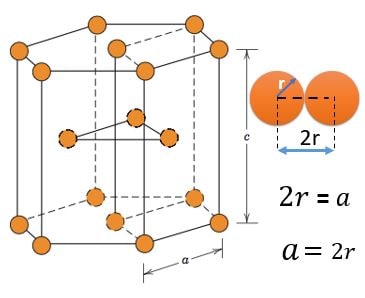
HCP (Hexagonal Closest Packed) :
Atom Positions :
(1) 12 Atoms, At every corner contributing \( \frac{1}{6} \) => 12 Corner atoms \( \times \) \( \frac{1}{6} \) contribution = 2 atom
(2) 2 Atoms, At top and bottom face center contributing \( \frac{1}{2} \) => 2 Face centered atoms \( \times \) \( \frac{1}{2} \) contribution = 1 atom
(3) 3 Atoms, Inside HCP structure contributing 1 => 3 atom
Total number of atoms = 2 + 1 + 3 = 6 atoms or 2 Atom per Unit (1HCP has 3 Unit structures )
 Notes :
Notes :
(1) HCP Elements are generally less ductile compare to FCC
(2) For regular HCP Element \( \frac{c}{a} = \sqrt{\frac{8}{3}} = 1.632 \).
(3) Examples :- Mg, Zn, Zr, Ti, Co.
Coordination Number :
CN is define as the total number of
nearest and equi-distance atoms surrounding an atom under consideration.
|
SC |
FCC |
BCC |
HCP |
DC |
| CN |
6 |
12 |
8 |
12 |
4 |
Atomic Packing Factor :
APF is define as the ratio of total avg. volume( Number of atoms \(\times \) total number of atom ) occupied by atoms to the volume of the unit cell.
\[ APF = \frac{N_{atoms} \times V_{atoms}}{V_{\textrm{unit cell}}} = \frac{N \times \frac{4}{3} \pi r^3}{a^3} \]
|
SC |
FCC |
BCC |
HCP |
DC |
| APF |
0.52 |
0.72 |
0.68 |
0.72 |
0.34 |
Generally, 3 type of densities are calculated in a crystal structure.
Volume Density or Theoratical Density or X-Ray Density :
\[ \rho_{v} = \frac{\textrm{Wt. of avg. no of atoms}}{\textrm{Volume of unit cell}} = \frac{Z \times M_{w}}{N_{A} \times a^3} \]
unit : \( \frac{g}{m^3} \)
Where ,
Z = Number of atoms in unit cell (atoms)
\(M_{w} = \) Molar Mass (g/mol)
\(N_{A} = \) Avogadro’s number = \( 6.023 \times 10^{23} \) \( \frac{atom}{mol} \)
V = Volume of unit cell = \( a^3 \) \( ( m^3 ) \)
Planer Density :
Planer Density is define as the Ratio of number of atoms who's centers are intersected by the plane to the area of the plane itself.
\[ \rho_{PL} = \frac{\textrm{Number of atomes in that plane}}{\textrm{Area of plane}} \]
unit : \( \frac{atoms}{m^2} \)
| Plane |
FCC |
BCC |
(100) |
\[ \frac{2}{a^2} \]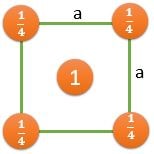 |
\[ \frac{1}{a^2} \]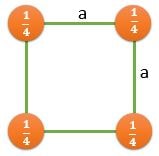 |
(110)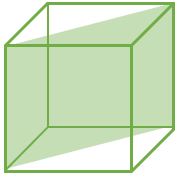 |
\[ \frac{2}{\sqrt{2}a^2} \]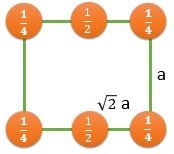 |
\[ \frac{2}{\sqrt{2}a^2} \textrm{ } (CPP) \]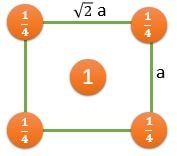 |
(111)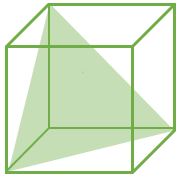 |
\[ \frac{2}{(\sqrt{3}/2)a^2} \textrm{ } (CPP) \]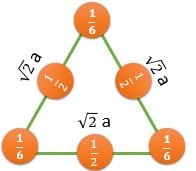 |
\[ \frac{1/2}{(\sqrt{3}/2)a^2} \] |
CPP (Closest Packed Plane): These planes have higher value of planer density. Hence, Slip occurs in such planes
For FCC : (111) Plane
For BCC : (110) Plane
Linear Density :
Linear density defined as the number of atoms whose centers are intersected by a line to the length of the line itself.
\[ \rho_{l} = \frac{\textrm{Number of atomes intersected by line}}{\textrm{Length of direction vector i.e, line}} \]
unit : \( \frac{atoms}{m} \)
| Direction |
FCC |
BCC |
| [100] |
\[ \frac{1}{a} \] |
\[ \frac{1}{a} \] |
| [110] |
\[ \frac{2}{\sqrt{2}a} \textrm{ } (CPD) \] |
\[ \frac{1}{\sqrt{2}a} \] |
| (111) |
\[ \frac{1}{(\sqrt{3})a} \] |
\[ \frac{2}{(\sqrt{3})a} \textrm{ } (CPD)\] |
CPD (Closest Packed Direction): These Directions have higher value of Linear density. Hence, Slip occurs in such direction
For FCC : [110] Direction
For BCC : [111] Direction
 Learn Metallurgy
Learn Metallurgy
















