 Learn Metallurgy
Learn Metallurgymail@learnmetallurgy.com
Potential - pH diagram, also called as Pourbaix diagram is a plot between equilibrium potential against pH of a electrochemical reactions .It shows the electrochemical stability for different states of an element as a function of pH at constant T and P.
Iron Pourbaix diagrams :
(1) \(Fe^{2+} + 2e^{-} \rightarrow Fe_{(s)} \) Slope = 0 i.e, indepecdent of PH
(2) \(Fe^{3+} + e^{-} \rightarrow Fe^{2+} \) Slope = 0 i.e, indepecdent of PH
(3) \(2Fe^{3+} + 3H_{2}O \rightarrow Fe_{2}O_{3} (s) + 6H^+ \) Slope = infinite i.e, independent of Potential
(4) \(Fe^{3+} + 3H_{2}O \rightarrow Fe_{2}O_{3} (s) + 6H^+ + 2e^{-}\) slope = -0.177
\( E = {E^o}_{H^+/H} -\frac{0.0591}{2} log \left[ \frac{1}{[H^+]^6} \right] \)
\( E = 0 + \frac{0.0591}{2} \times 6 log [H^+] \)
\( E = -3 \times0.0591 pH = -0.177pH \)
(5) \( Fe_{3}O_{4} (s)+ H_{2}O \rightarrow 2H^+ + 2e^{-}\) slope = -0.0591
Application of pourbaix diagram :
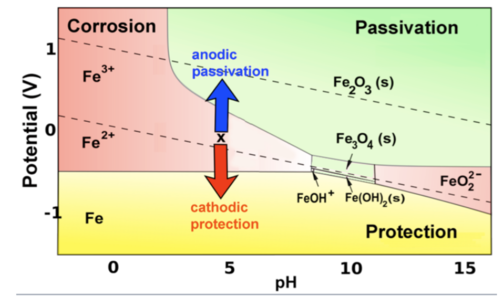
(1) Domain of immunity / Protection :
In this domain, Metal is the stable species and it will be protected from corrosion. In Cathodic protection (ICCP) we supply external current to bring down potential to this domain.
(2) Domain of corrosion :
In this domain, metal soluble ion is the stable species and metal tends to dissolve to the solution in this domain i.e, corrosion will occure if kinetics are favourable.
(3) Domain of passivation :
In this domain, insoluble metal oxides are stable species that can protect the metal if it forms animpervious adherent layer. In anodic protction, polarization is done to bring metal to this domain so that a passive layer can from over the surface which will protect it from corrosion.
Limitations of pourbaix diagram :
(1) No kinetic infomation is given as these diagrams are derived from thermodynamic consideration
(2) Passivity domain does not tell about nature , adherence and coherence of the passive film. But can be measure through PB ratio.
(3) Pourbaix diagram does not take consideration of environmetals factor such as presence of cl or sulphate ions which can act as a catalyst for corrosion.
(4) Dependent on T. As this diagram is constructed using nernst equation which makes it temperature dependent