Also known as age-hardening, the hardness of a quenched alloy increases as a function of ageing time.
When a material is cooled slowly. First, the precipitaes will start nucleating at the triple junction and grain boundaries to reduce the energy. And this precipitates are brittle in nature and eventually metal gets fracture along the grain boundary. This can be prevented by rapid cooling which forms super-saturated solid solution, and on heating again the precipitate will nucleate inside the grains instead of grain boundary as diffusion is limited ( because of low temperature ). And with time these precipitates grow and restrict dislocation motion, hence materials strength increases with time( i.e., aging).
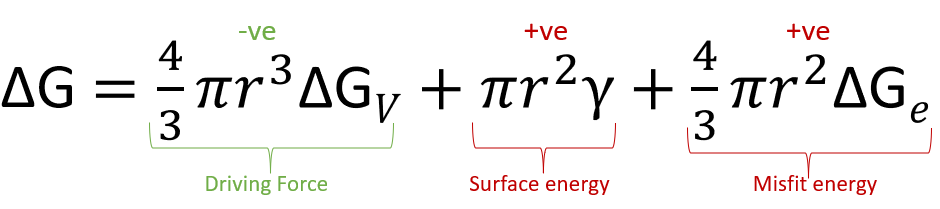
For solid - solid transformation, misfit strain between precipitate and the matrix also becomes important factor. And to reduce misfit energy \( \delta G_{e} \), precipitation will prefer locations as -
Surface > Triple-junction > Grain-boundary > stacking-fault > dislocation > vacancy
But when we do fast cooling then there won't be sufficient time for solute atom to go at the grain boundary and, form super-saturated solid solution.. And again on heating, it will start nucleating and precipitate inside the grains.
Basic requirements for precipitation strengthening :
-
Sloping solvus :
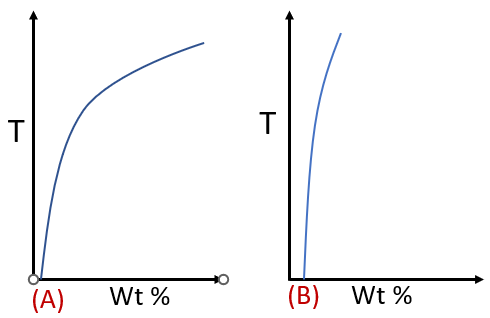
The solid solubility limit should decrease with decrease in temperature. i.e.,solvusline show in below figure (A) is more preferable. As the supersaturation is the driving force for the rejection of the excess solute in the form of precipitates. In case A, precipitation will be much more.
-
Coherency of initial precipitate :
As we have seen in the above formula, that the total \( \Delta G \) will depend on the misfit strain energy. So the precipitate of second phase shold be coherent in nature (i.e., less misfit energy). But later on, as the precipitate size increases the loss of coherency occure at the interface of precipitate and matrix.
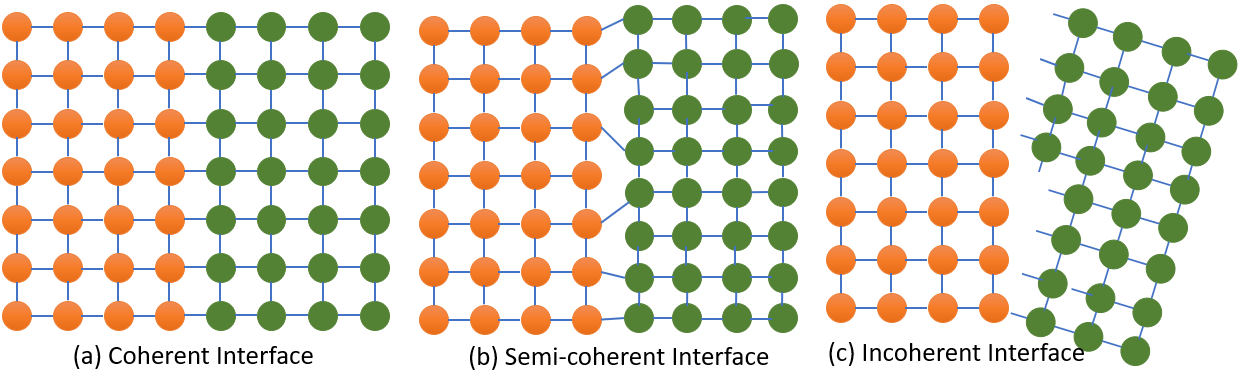
Coherent interface : There is a one-to-one matching of the lattice planes across the interface. The lattice parameter of matrix and precipitae is almost equal, this generally produces a strain known as coherency strain and imposing very low misfit strain.
Semi-coherent interface : There not a little bit matching of the lattice planes across the interface. And the misfit strain will be higher than coherent interface.
In-coherent interface : There not a single matching of the lattice planes across the interface. And the misfit strain will be highest at this interface.
-
Precipitate strength :
The amount and strength of the solute should be sufficient to impart strength to the matrix.
Steps in Age - hardening treatment

-
Solutionizing :
It is the process of heating the alloy just above the solvus temperature but below its melting point to obtain a single phase solid solution. In this step all the precipitate will get dissolve as solubility at higher temperature is high and thus form a single phase solid solution.

-
Quenching : The solutionized alloy is cooled rapidly to retain the high temperature single phase solid solution at room temperature as metastable supersaturated solid solution (SSSS). Here, solute atoms won't have sufficient time to diffuse towards grain boundary so they trapped inside the grain in single phase.

-
Ageing :
Inthis step, we heat the SSSS to an elevated temperature for controlled decomposition of SSSS to form finely dispersed precipitates.
Natural ageing : Ageing is done by holding the quenched alloy at room temperature for longer time
Artificial ageing : Ageing is done by holding the quenched alloy at slightly higher temperature than room temperature and it takes less time than the natural ageing.
At any given temperature, with time (or ageing) finely-dispersed-precipitates will start forming and then precipitates will start growing in size at the cost of dissolution of smaller precipitates to reduce the total surface energy, hence, \( \Delta G \). which increases the hardness. As the precipitation continues, precipitate gets coarsen and become brittle which reduces its strength, which is known as coarsening.

The hardness will initially increase and then decreases after achieving peak-hardness. Peak hardness will be less at higher ageing temperature (T1) but peak hardness will take less time than lower temperature (T2)
| # |
Precipitation |
coarsening |
| 1 |
The average precipitate size increases with time because of temperature |
The average precipitate size increases because of Gibbs-Thomson effect (i.e., large precipitaes grow at the expense of smaller precipitates |
| 2 |
Total number of precipitates increases with ageing time |
Number of precipitates decreases as smaller precipitates get dissolved |
| 3 |
The inter-precipitate distance increases as number of precipitates increases |
The inter-precipitate distance decreases as number of precipitates decreases |
| 4 |
Total volume of precipitates will increase |
The total volume of precipitates remains constant with time |
Age - hardening treatment of duraluminum alloy ( Al-4.5% Cu)
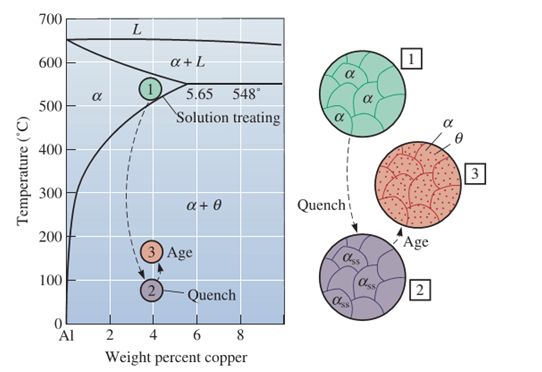
The above figure shows the age-hardening process for Al-Cu alloy. The annealed alloy is first solutionize (heating above 550 oC) to form single phase \( \alpha \) and then quenched to obtain SSSS. The decomposition of SSSS during ageing is a bit complex process. The equilibrium precipitate \( \theta \) Al2Cu, normally does not form directly from the SSSS because of the large nucleation barrier for direct transformation \( \Delta G_1 > \Delta G_2 \). Instead the precipitation occures in several steps involving several transition (metastable) precipiotate before it forms the equilibrium precipitate. The precipitation sequence for SSSS of Al-4.5%Cu alloy follows as -
\[ \textrm{GP Zones} \rightarrow \theta^{``} \rightarrow \theta^{`} \rightarrow \theta \]
Here, three distinct precipitates will form and dissolve prior to the formation of the equilibrium-precipitate (\( \theta \)), As described earlier, this sequence occurs because the equilibrium-precipitate (\( \theta \)), is incoherent with the matrix, where as the metastable precipitates like GP-Zones and (\( \theta ^{``} \)) are fully coherent with the matrix, and (\( \theta ^{`}\)), is semicoherent. Hence, the system follows such sequence so that it has lowest free energy in all the stages of precipitation.
-
GP Zones
Guinier-Preston zones or GP zones form during the early stage of ageing. GP zones are coherent in nature and have the same crystal structure (FCC) and lattice parameter the \( \alpha_{SSSS}\). They only differ in composition that is why formation of GP zones is preferred over \( \theta \). GP zones are plate-like clusters predominantly of copper atoms segregated on to {100} planes of the aluminium lattice.
-
\( \theta ^{``}\) Precipitate
Also called as GP-2 zones, it has a different crystal structure than matrix. This phase is also coherent in nature. Formation of this precipitate result in increase in hardness. As coherency strain field increases around the precipitate.
-
\( \theta ^{`} \) Precipitate
This transition precipitate is disc-shaped and semi-coherent with the matrix. It has a tetragonal structure and formation of \( \theta^{`} \) precipitate leads to the softening of the alloy and fomation of these precipitaes leads to decrease in hardness.
-
\( \theta \) Precipitate
This is the equilibrium precipitate \( \theta \) (Al2Cu), has a tetragonal structure. it is fully incoherent precipitate and thus its formation always leads to softening as coherency strain becomes zero. \( \theta \) precipitates are the ultimate result of overageing.

The maximum hardness in an alloy is obtained when there is an intermediate precipitate ( \( \theta^{``} \textrm{ or } \theta^{`} \) ). After the peak hardness, further ageing tends to decrease the hardness i.e., overageing
 Learn Metallurgy
Learn Metallurgy








