 Learn Metallurgy
Learn Metallurgymail@learnmetallurgy.com
A materials strength can be improve by adding different soluble elements to the parent matrix (i.e., alloying), which impede the movement of dislocations in the crystal.
The fact that the alloys are stronger than the pure metal is because of the addtion of solute atoms. Solute atoms can form either a substitution solid solution or interstitial solid solution in a sovent/parent metal and act as atomic-sized obstacles to the motion of dislocation. Hence, solid solutions offers more strength than pure metals.
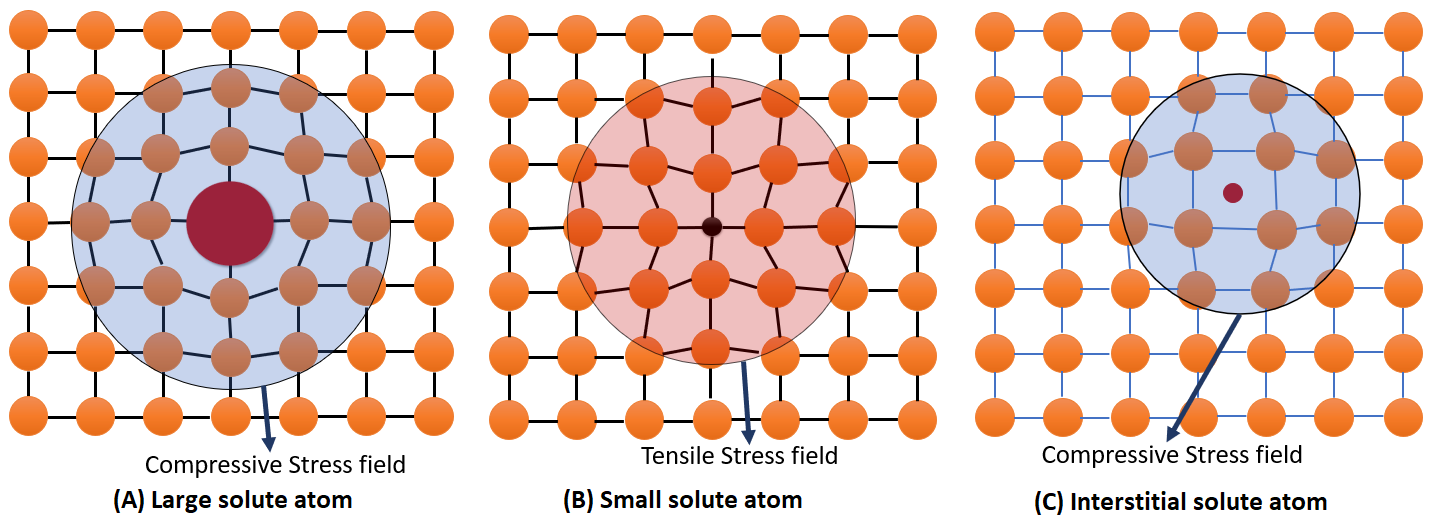
In general, the size of solute atom can be larger or smaller than the parent atom. Large solute atom creates a compresive stress field around it, small solute atom create a tensile stress field around it and an interstitial atom create a compressive stress field. This can be easily visualize be thinking about the force felt by the bonds to maintain same bond length.
On addition of solute atom, the solute atom sits near the dislotion to redure strain-energy. Solute-dislocation interaction locks dislocation and couse immobilization or pinning of the dislocation. For example large solute will sit near the tension side of edge dilocation and similarly, smaller atom will sit near the compressive side of edge dislocation, which enentually balances the both stress field created by dislocation as well as solute atom. Which results in lowering the strain energy.
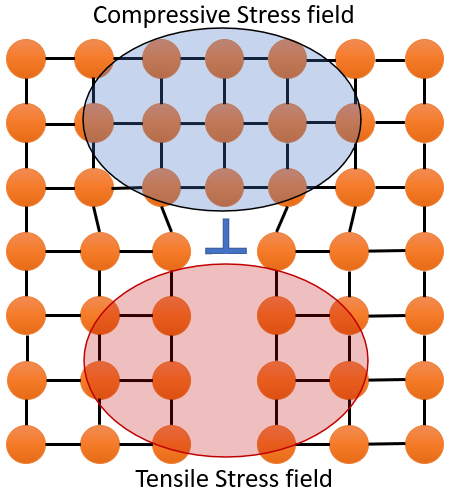
Now, when external stress is applied then solute atom restrict the motion of dislocation and hence material strength increases.
Humes-Rohtery shows the factors which control the tendency for the fomation of substitutional solid solution are -
| # | Solid-Solution | Compound |
|---|---|---|
| 1 | SS have no fixed composition. The concentration is a variable | Compound have fixed solute concentration |
| 2 | Can't be expressed by formula | Compounds have a chemical formula |
| 3 | Solid solution melt or solidify over a range of temperature | Compound melt or solidify at a constant temperature like a pure element |
| 4 | Crystal structure of SS is same as the parent element | Crystal structure can be differ from either of the components. |
| 5 | Not chemically bonded | In compound atoms are chemically bonded to each other. |
| 6 | Example : Ferrite, Austenite, Brass | Fe3C, NaCl |